Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Suzuki, Satoru; Sato, Haruo; Ishidera, Takamitsu; Fujii, Naoki*; Kawamura, Katsuyuki*
JNC TN8400 2001-031, 44 Pages, 2002/05
In order to quantify effect of temperature on diffusivity of deuterated water (HDO) in compacted sodium-bentonite, through-diffusion experiments were conducted at elevated tempemture from 298 to 333 K. Kunipia F (Na-montmorillonite content  98 wt. %; Kunimine Industly Co.) was compacted to a dry density of 0.9 and l.35 Mg/m
98 wt. %; Kunimine Industly Co.) was compacted to a dry density of 0.9 and l.35 Mg/m . Since smectite flakes were perpendicularly oriented to a direction of compaction, anisotropy of diffusivity was investigated parallel and normal to the preferred orientation of smectite. Effective diffusion coeficient D
. Since smectite flakes were perpendicularly oriented to a direction of compaction, anisotropy of diffusivity was investigated parallel and normal to the preferred orientation of smectite. Effective diffusion coeficient D of HDO was larger for a diffusional direction parallel to the preferred orientation than normal to that for both dry densities. These results well agreed to the previously reported ones for tritiated water. Activation energies of D
of HDO was larger for a diffusional direction parallel to the preferred orientation than normal to that for both dry densities. These results well agreed to the previously reported ones for tritiated water. Activation energies of D in compacted bentonite increased with increasing dry density in the range of 19 - 25 kJ/mol which was slightly larger than that in bulk water (18 kJ/mol). This relationship can be considered to be due to both the pore structure development and high activation energy of water (18-23 kJ/mol) in the vicinity of smectite surface (within 2 nm) on the basis of molecular dynamics simulations.
in compacted bentonite increased with increasing dry density in the range of 19 - 25 kJ/mol which was slightly larger than that in bulk water (18 kJ/mol). This relationship can be considered to be due to both the pore structure development and high activation energy of water (18-23 kJ/mol) in the vicinity of smectite surface (within 2 nm) on the basis of molecular dynamics simulations.
Onuki, Toshihiko; Kozai, Naofumi; Samadfam, M.; Yamamoto, Shunya; Narumi, Kazumasa; Naramoto, Hiroshi; Kamiya, Tomihiro; Sakai, Takuro; Murakami, Takashi*
Nuclear Instruments and Methods in Physics Research B, 181(1-4), p.644 - 648, 2001/07
Times Cited Count:4 Percentile:33.81(Instruments & Instrumentation)no abstracts in English
Suzuki, Satoru; Kawamura, Katsuyuki*
JNC TN8400 2001-005, 41 Pages, 2001/04
A correlation between molecular structure and a vibrational spectrum of interlayer water in Na-smectite was investigated by means of Molecular Dymamics (MDs) simulations. Detailed comparison of simulation results with IR spectroscopic observations for the water-smectite system indicated good agreement. Internal vibrational spectra of water were obtained by the Fourier transformation of velocty auto-correlation function of hydrogen atom. A stretching vibrational spectrum of interlayer water consisted of a broad band with a peak top around 3400cm and a sharp peak around 3650 to 3700cm
and a sharp peak around 3650 to 3700cm . The fomer broad band was assigned to O-H vibrations between water molecules as bulk water, while the latter band was attributed to O-H ones oriented to siloxane surface through hydrogen bonding. The hydrogen bond distance, determined as the shortest O-O distance by the radial distribution function (RDF), revealed that hydrogen bond distance between water and siloxane surface (O
. The fomer broad band was assigned to O-H vibrations between water molecules as bulk water, while the latter band was attributed to O-H ones oriented to siloxane surface through hydrogen bonding. The hydrogen bond distance, determined as the shortest O-O distance by the radial distribution function (RDF), revealed that hydrogen bond distance between water and siloxane surface (O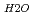 -O
-O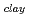
 3.0
3.0  -O
-O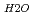 = ca. 2.8 AA ). These results suggested that interaction between water molecule and siloxane surface weaker than that between water molecules, although they were forced to be oriented.
= ca. 2.8 AA ). These results suggested that interaction between water molecule and siloxane surface weaker than that between water molecules, although they were forced to be oriented.
Oda, Chie; Ikeda, Takao*; Shibata, Masahiro
JNC TN8400 99-073, 112 Pages, 1999/11
In the safety assessment for geological disposal of high-level radioactive wastes (HLW), distribution coefficients (Kd) and diffusion coefficients of radionuclides are used to estimate the migration of radionuclides in a near-field of repository.  Sn is one of the important nuclides for the safety assessment in Japan and its behavior under reopsitory conditions has not been understood. This report provides the experimental informations for the sorption of Sn on bentonite, tuff and granodiorite, and the diffusion of Sn in a compacted bentonite. The Kd values of Sn on bentonite, tuff and granodiorite were determined by the batch-type sorption experiments as l0
Sn is one of the important nuclides for the safety assessment in Japan and its behavior under reopsitory conditions has not been understood. This report provides the experimental informations for the sorption of Sn on bentonite, tuff and granodiorite, and the diffusion of Sn in a compacted bentonite. The Kd values of Sn on bentonite, tuff and granodiorite were determined by the batch-type sorption experiments as l0
 10
10 [ml/g], 10
[ml/g], 10
 10
10 [ml/g] and 10
[ml/g] and 10
 10
10 [ml/g], respectively. The sequential extraction experiments for adsorbed Sn on bentonite were also performed to investigate its desorption behavior. These experimental results indicated that the mechanisms of sorprion onto bentonite were dominated by the sorption reactions on smectite and pyrite and consisted of reversible and irreversible sorption on solid and stable fixation in solid. On the other hands, the apparent diffusion coefficients (Da) in compacted bentonite were measured by the diffusion experiments as 10
[ml/g], respectively. The sequential extraction experiments for adsorbed Sn on bentonite were also performed to investigate its desorption behavior. These experimental results indicated that the mechanisms of sorprion onto bentonite were dominated by the sorption reactions on smectite and pyrite and consisted of reversible and irreversible sorption on solid and stable fixation in solid. On the other hands, the apparent diffusion coefficients (Da) in compacted bentonite were measured by the diffusion experiments as 10 [m
[m /sec] and l0
/sec] and l0 [m
[m /sec] for dry densities of 0.4[g/cm
/sec] for dry densities of 0.4[g/cm ] and 1.0[g/cm
] and 1.0[g/cm ], respectively. Moreover, the Kd values in compacted bentonite were calculated according to the relationship with the measured Da values, and the solubilities in the porewaters of compacted bentonite were calculated by use of the calculated Kd and the obtained diffusion plofiles. It is found that the derived solubilities almost agreed with the solubiliies of amorphis SnO
], respectively. Moreover, the Kd values in compacted bentonite were calculated according to the relationship with the measured Da values, and the solubilities in the porewaters of compacted bentonite were calculated by use of the calculated Kd and the obtained diffusion plofiles. It is found that the derived solubilities almost agreed with the solubiliies of amorphis SnO reported by Amaya et al. (1997), however, the derived Kd values were lower than that measured from the batch-type sorption experiments.
reported by Amaya et al. (1997), however, the derived Kd values were lower than that measured from the batch-type sorption experiments.
Futakuchi, Katsuhito*; Sakuramoto, Yuji*; *; *; *; Hashimoto, Shuji*
PNC TJ1308 98-001, 103 Pages, 1998/02
None
Sato, Haruo
PNC TN8410 97-202, 205 Pages, 1998/01
This thesis summarizes the results investigated in order to establish a basic theory on the predictive method of diffusion coefficients of nuclides in compacted sodium bentonite which is a candidate buffer material and in representative rocks for the geological disposal of radioactive waste by measuring the pore structural factors of the compacted bentonite and rocks such as porosity and tortuosity, measuring diffusion coefficients of nuclides in the bentonite and rocks, acquiring basic data on diffusion and developing diffusion models which can quantitatively predict nuclide migration in long-term. This consists of 7 chapters. Chapter 1 is the introduction, in which conventional studies on nuclide migration in buffer materials and rocks for the geological disposal of radioactive waste carried out to date are reviewed, and those problems are summarized as well as the objectives of this study are described. Besides, the difinition of geological disposal is explained. In Chapter 2, it is described on non-steady state diffusion of HTO, Sr-90, Tc-99, I-129, Cs-137, Np-237, Am-241 and Pu in purified sodium bentonite, Kunipia-F, in which the rate of constituent Na-smectite was raised approximately 100wt%. In-diffusion experiments were carried out in a range of bentonite densities of 200  2000 kg
2000 kg m
m under ambient aerobic conditions at room temperature (20
under ambient aerobic conditions at room temperature (20  23
23 C), and apparent diffusion coefficients (Da) were obtained. The apparent diffusion coefficients decreased with increasing dry density of bentonite. It was quantitatively indicated from diffusion experiments using HTO that these Da values include the effect of geometric retardation such as the tortuosity factor of compacted bentonite. It was experimentally clarified that Da is not affected by diffusion time based on diffusion experiments for different experimental periods using Sr and Cs. Moreover, it was also experimentally clarified that Da is not affected by tracer ...
C), and apparent diffusion coefficients (Da) were obtained. The apparent diffusion coefficients decreased with increasing dry density of bentonite. It was quantitatively indicated from diffusion experiments using HTO that these Da values include the effect of geometric retardation such as the tortuosity factor of compacted bentonite. It was experimentally clarified that Da is not affected by diffusion time based on diffusion experiments for different experimental periods using Sr and Cs. Moreover, it was also experimentally clarified that Da is not affected by tracer ...
Sato, Tsutomu
Sumekutaito Kenkyukai Kaihou, 8(2), p.44 - 46, 1998/00
no abstracts in English
Sato, Tsutomu
Sumekutaito Kenkyukai Kaihou, 7(2), p.2 - 13, 1997/11
no abstracts in English
Sato, Tsutomu
Sumekutaito Kenkyukai Kaihou, 7(2), p.39 - 41, 1997/00
no abstracts in English
Sato, Tsutomu; Murakami, Takashi*; Watanabe, Takashi*
Clays and Clay Minerals, 44(4), p.460 - 469, 1996/00
Times Cited Count:37 Percentile:75.43(Chemistry, Physical)no abstracts in English
Kozai, Naofumi; Onuki, Toshihiko; Matsumoto, Junko; Bamba, Tsunetaka; *
Radiochimica Acta, 75(3), p.149 - 158, 1996/00
no abstracts in English
Sato, Tsutomu; Murakami, Takashi*; Isobe, Hiroshi; Onuki, Toshihiko
Materials Research Society Symposium Proceedings, Vol.353, 0, p.239 - 246, 1995/00
no abstracts in English
Sato, Tsutomu
SMECTITE, 4(1), p.48 - 51, 1994/05
no abstracts in English
Sato, Tsutomu
Nihon Genshiryoku Gakkai-Shi, 36(5), p.405 - 412, 1994/00
Times Cited Count:0 Percentile:0.01(Nuclear Science & Technology)no abstracts in English
Kozai, Naofumi; Onuki, Toshihiko; Muraoka, Susumu
Nihon Genshiryoku Gakkai-Shi, 36(10), p.955 - 957, 1994/00
Times Cited Count:2 Percentile:28.09(Nuclear Science & Technology)no abstracts in English
Onuki, Toshihiko; Murakami, Takashi; Sato, Tsutomu; Isobe, Hiroshi
Radiochimica Acta, 66-67, p.323 - 326, 1994/00
no abstracts in English
Onuki, Toshihiko; Kozai, Naofumi
Radiochimica Acta, 66-67, p.327 - 331, 1994/00
no abstracts in English
Kozai, Naofumi; Onuki, Toshihiko; Muraoka, Susumu
Journal of Nuclear Science and Technology, 30(11), p.1153 - 1159, 1993/11
Times Cited Count:26 Percentile:89.52(Nuclear Science & Technology)no abstracts in English